Abstract
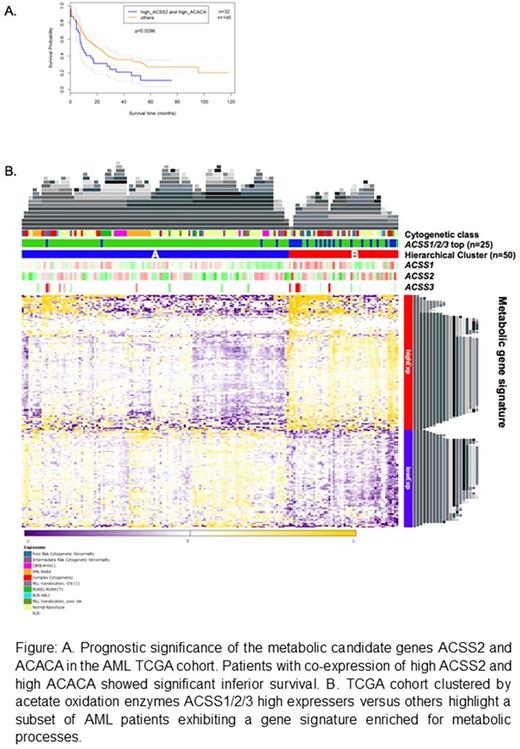
Determining the cellular features that contribute to chemotherapy resistance in acute myeloid leukemia (AML) has been rigorously investigated to improve therapeutic options. Leukemia cell crosstalk with bone marrow-derived mesenchymal stroma is one of the major sources of drug resistance considering stromal cells provide growth factors, extracellular matrix, and metabolites meanwhile facilitating the protection of leukemia cells to chemotherapeutic agents. Here, we modeled leukemia/stroma crosstalk on a global scale to uncover activated signaling pathways through mass spectrometry-based phosphoproteomics. Coculture of KG1a leukemia with Hs5 stroma cells exhibiting potent stroma protective effects (SPE) were promoted by treatment with the HDAC inhibitor apicidin (1-10µM), whereby leukemia apoptosis levels decreased by 40-50% in the presence of stroma compared to monocultured KG1a cells. KG1a and Hs5 cocultures exhibiting SPE were then profiled for phosphorylation events to unravel SPE which resulted in detection of 683 differentially phosphorylated peptides mapping to activated pathways involved in MAPK, AMP and cytoskeletal, and metabolic pathways. Notably, top differentially ranked phosphosites, acetyl-coenzyme A synthetase (ACSS2, Ser30) and acetyl-CoA carboxylase alpha (ACACA, S80) were hyper and hypophosphorylated, respectively, in cocultured leukemia/stroma relative to the monocultures of leukemia and stroma. ACSS2, catalyzing fatty acid (FA) synthesis, was further investigated through inhibition of the FA pathway with compound AZ62, a fatty acid synthase (FASN) inhibitor that was previously reported to effectively reduce solid tumor growth and inhibit FA synthesis (1). Targeting the FA pathway deemed leukemia sensitive to AZ62 (1.2-5ng/µl) with increased apoptosis (>50%) in both leukemia cell lines and primary AML blasts (n=5). We then further hypothesized that acetate, a substrate of ACSS2, was a plausible metabolite for mediating SPE, whereby excess acetate released by the stroma allowed for leukemia uptake. To elucidate the role of acetate uptake, acetate (6-12.5μM) was exogenously added to leukemia cells, which improved leukemia cell viability (1.2-1.4 fold) in KG1a, OCI-AML3 cells as well as in primary AML blasts (1.2 -1.4 fold, n=7). To further assess the role of ACSS2 and ACACA in AML, mRNA expression of both candidates were analyzed in the AML TCGA cohort (n=172). We found that the status of high ACSS2 and high ACACA co-expressers (n=32) versus others (n=140) was predictive of poor overall survival (8.9 versus 20.5 months, P-value=0.03). An independent cohort, Metzeler et al. dataset, was used to validate this result (P-value=0.006). We then hypothesized that enzymes mediating acetate oxidation were potentially involved in AML thus we identified high expressers of acetate oxidation enzymes (ACSS1/2/3) and found a subset of patients with a gene signature enriched for metabolic processes. Similarly, high ACSS1/2 expressers versus others also displayed a metabolic signature in the Metzeler et al cohort. Taken together, these results report: 1) the phosphoproteomic dataset reveal phosphorylation of major signaling pathways with inference to the protein signaling activated by the stroma protection of leukemia cells. 2) ACSS2 may mediate SPE with acetate as a possible key metabolite in enhancing leukemia cell survival. 3) These data shed light on the role of metabolic processes in the pathogenesis of AML defining a subset of patients with a distinct metabolic signature that may serve as resource for improving leukemia drug discovery.
1. Schug ZT et al . Cancer Cell. 2015; 27: 57-71.
2. TCGA. Nat. Genet. 2013; 45:1113-20.
3. Metzeler KH et al . Blood 2008; 112: 4193-4201.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.